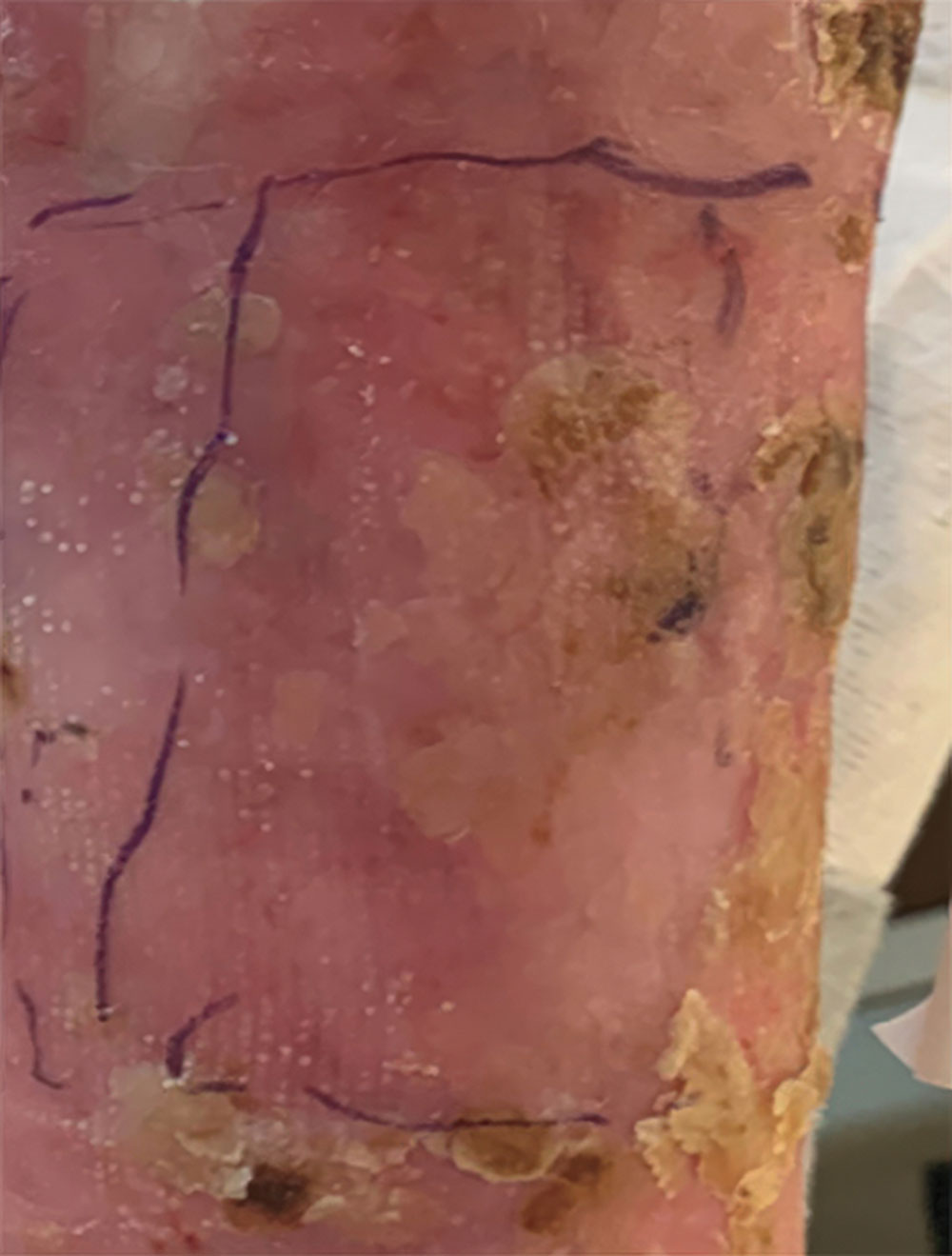What is ZEVASKYN?
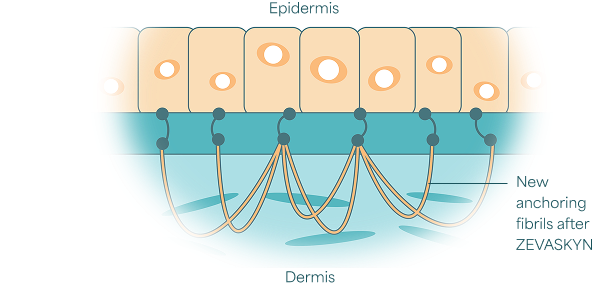
ZEVASKYN is designed for long-lasting wound healing with a single surgical application
What is it?
ZEVASKYN (ZEE-vah-skin) gene-modified cellular sheets are made from your own skin cells and surgically applied.
How is it made?
Two punch biopsies are taken from your skin and sent to Abeona Therapeutics®. The cells are then genetically modified to produce working type VII collagen. Over a few weeks, the cells are grown into credit card–sized ZEVASKYN cellular sheets.
How is it applied?
In a single surgery, ZEVASKYN sheets are applied to your wounds under general or other appropriate anesthesia.
How does it work?
ZEVASKYN works by producing functional type VII collagen in your treated wounds. This forms anchoring fibrils that bond together the epidermal and dermal layers of your skin.
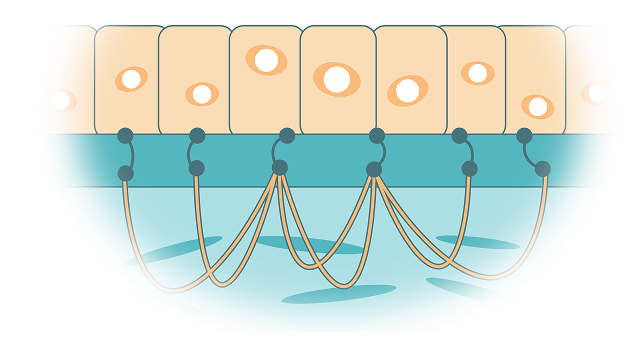
How much wound coverage can ZEVASKYN provide?
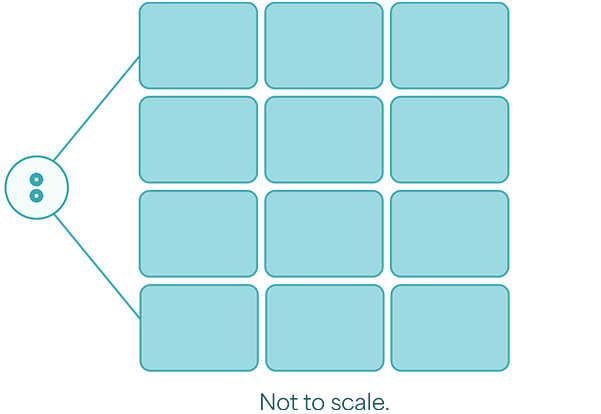
Your two 8 mm punch biopsies (the size of 2 small peas) expand up to 12 credit card–sized sheets.
ZEVASKYN provides unprecedented coverage for RDEB wounds in a single surgical session
In a clinical study, patients with wound size of 20 cm2 or more were treated with ZEVASKYN sheets in one surgery under general or other appropriate anesthesia.
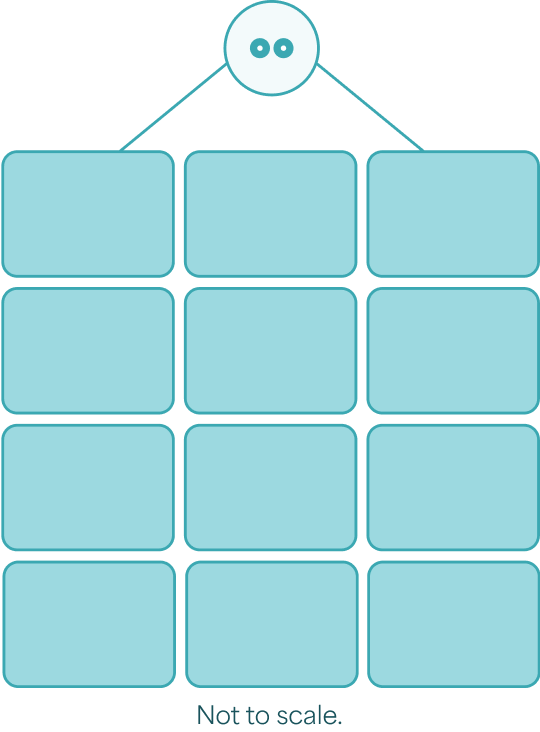
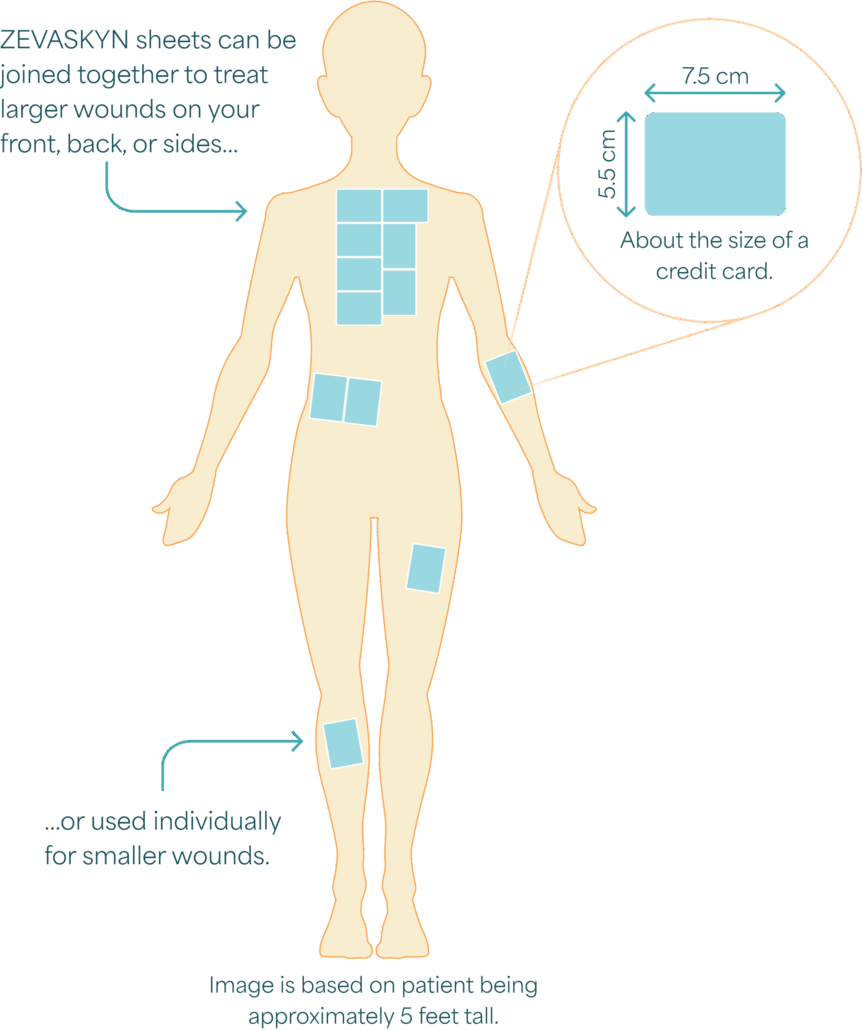
You can receive up to 12 credit card–sized ZEVASKYN sheets in one surgical session.
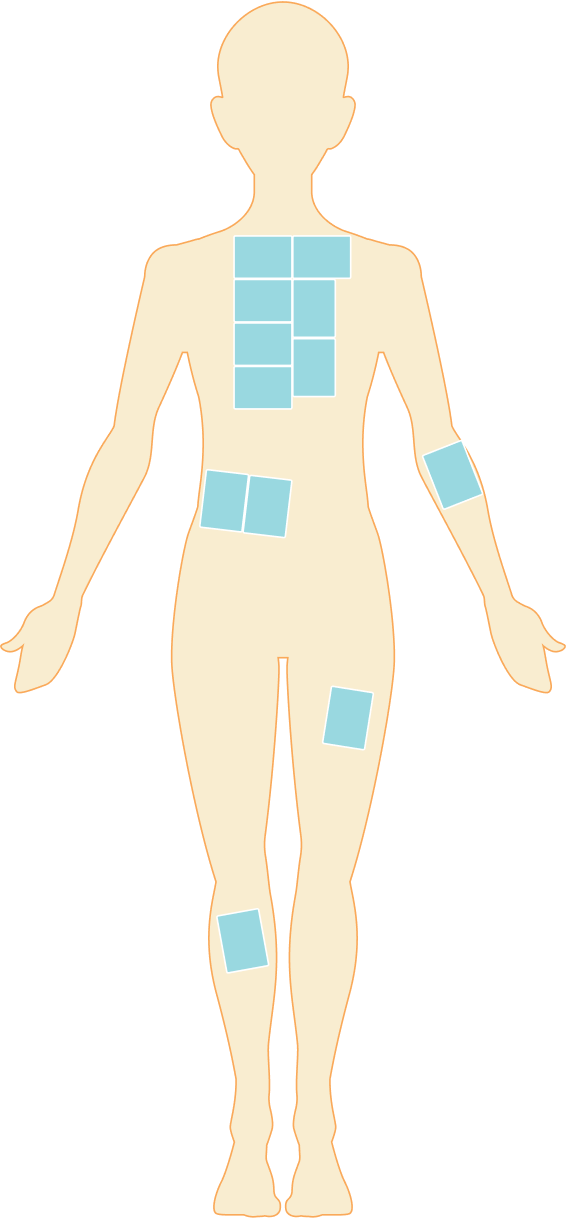
Image is based on patient being approximately 5 feet tall.
You can receive up to 12 credit card–sized ZEVASKYN sheets in one surgical session.
How was ZEVASKYN studied?
ZEVASKYN was studied in chronic RDEB wounds of various shapes and sizes
A clinical study (VIITAL) assessed how well ZEVASKYN works
The clinical study evaluated 43 ZEVASKYN-treated wounds in 11 patients over 6 months. Each wound was compared to a matched control wound of similar size and chronicity in the same patient.* A total of 86 wounds were evaluated across all patients.
- Ages of patients treated in the study ranged from 6 to 40 years old
- Wounds studied had been open for a median (middle value) of 5 years with a range of 6 months to 21 years†
- Wound size had to be at least 20 cm2
- Patients in the clinical study received a median of 6 ZEVASKYN sheets (range of 3-6 sheets), which was the maximum number allowed in the clinical study†
At 6 months, the study measured:
- Wound healing
- Pain reduction
- Itch reduction
- *43 control wounds (those not treated with ZEVASKYN) received standard of care treatment (eg, bandages and palliative care).
- †Median is the middle number in a group of numbers arranged from lowest to highest.
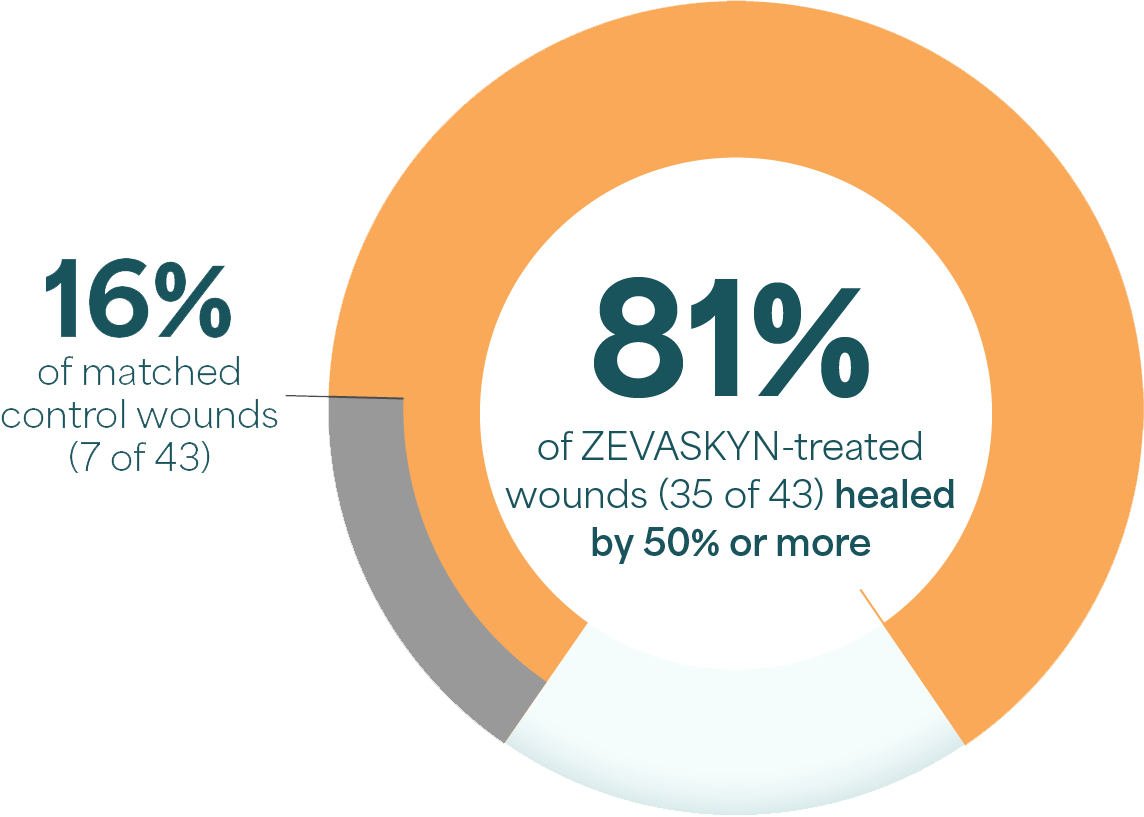
Significant wound healing with ZEVASKYN at 6 months
- Complete healing seen in 16% of ZEVASKYN-treated wounds vs none of the matched control wounds‡
- ~2 out of 3 ZEVASKYN-treated wounds (28 of 43) healed by 75% or more vs 7% of matched control wounds (3 of 43)
ZEVASKYN-treated wounds showed noticeable healing within 6 weeks, compared to fewer control wounds. This analysis was based on a subset of wounds (28/43) that were assessed at 6 weeks.§
- ‡Complete wound healing was defined as the new skin completely covered, with no drainage, no damage of the treated skin, and no major scabbing.
- §Not all wounds were evaluated early at 6 weeks. The study was not specifically designed to fully measure these results, so they should be interpreted carefully.
ZEVASKYN: Proven to reduce pain in treated wounds
At 6 months (VIITAL study)
- ||Pain reduction was measured on a 0-10 scale before and after treatment (following wound dressing change). Pain reduction was measured using the Wong-Baker FACES® Pain Rating Scale that is used to help people communicate the severity of their pain by choosing a face that best matches their experience.
- ¶Itch severity was measured using the Worst Itch-Numeric Rating Scale (WI-NRS), ranging from 0 (no itch) to 10 (worst itch imaginable).

Oh, it was bad [before treatment with ZEVASKYN]. It was constantly itching, and the medicine wasn’t helping. And then [after treatment with ZEVASKYN, there] was less itching, less wounds, and less pain, too. When it heals [after surgery], I don’t feel itching.”
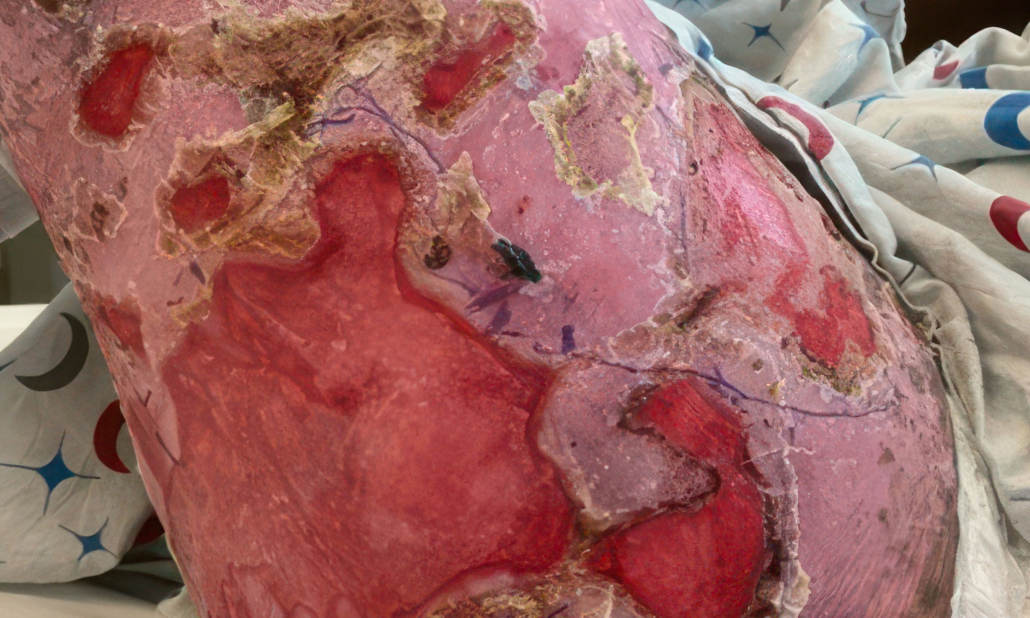
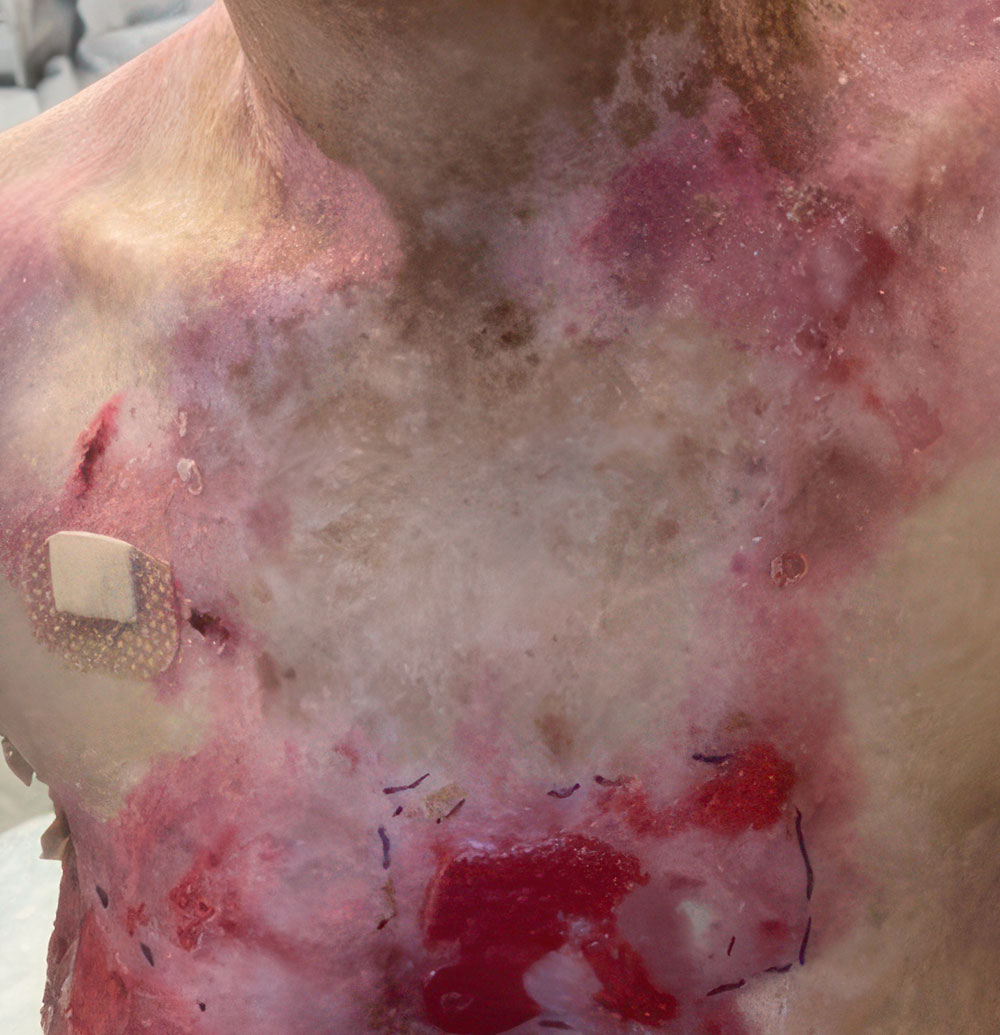
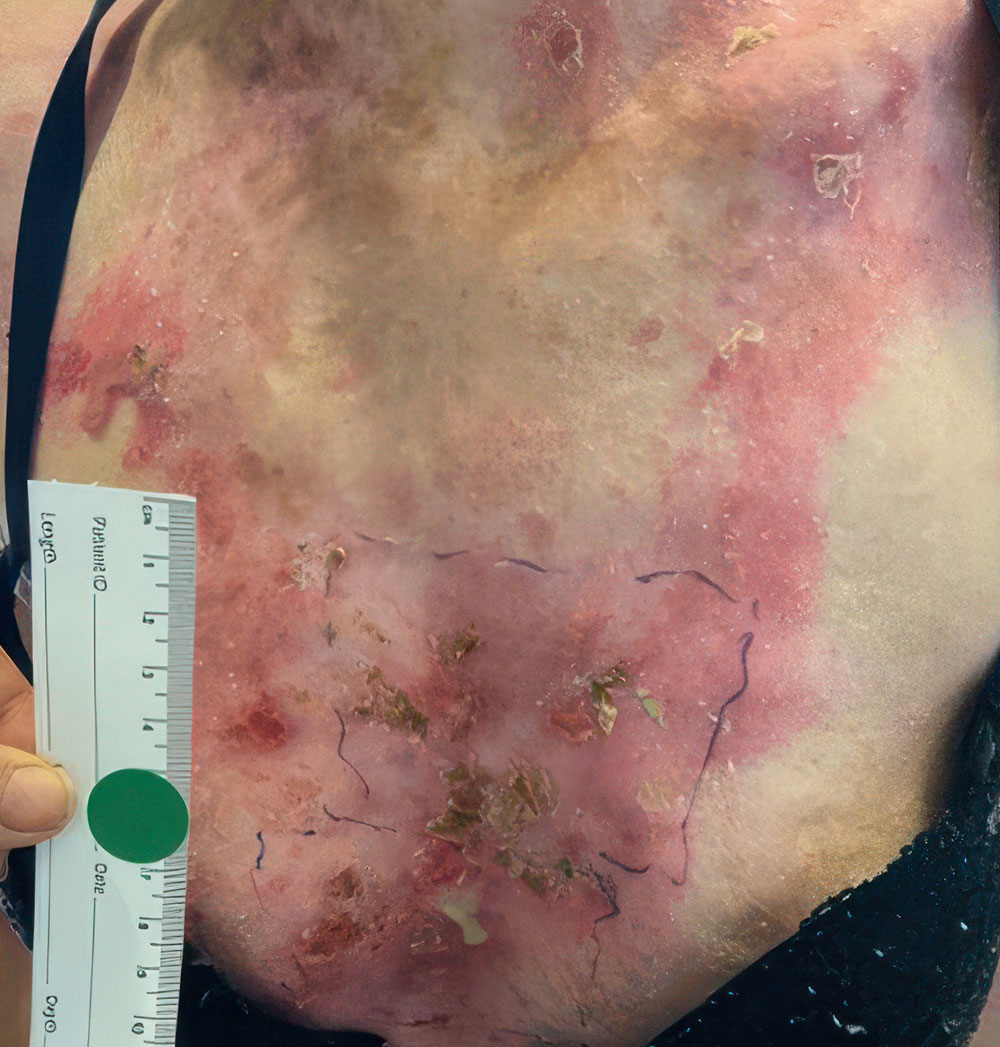
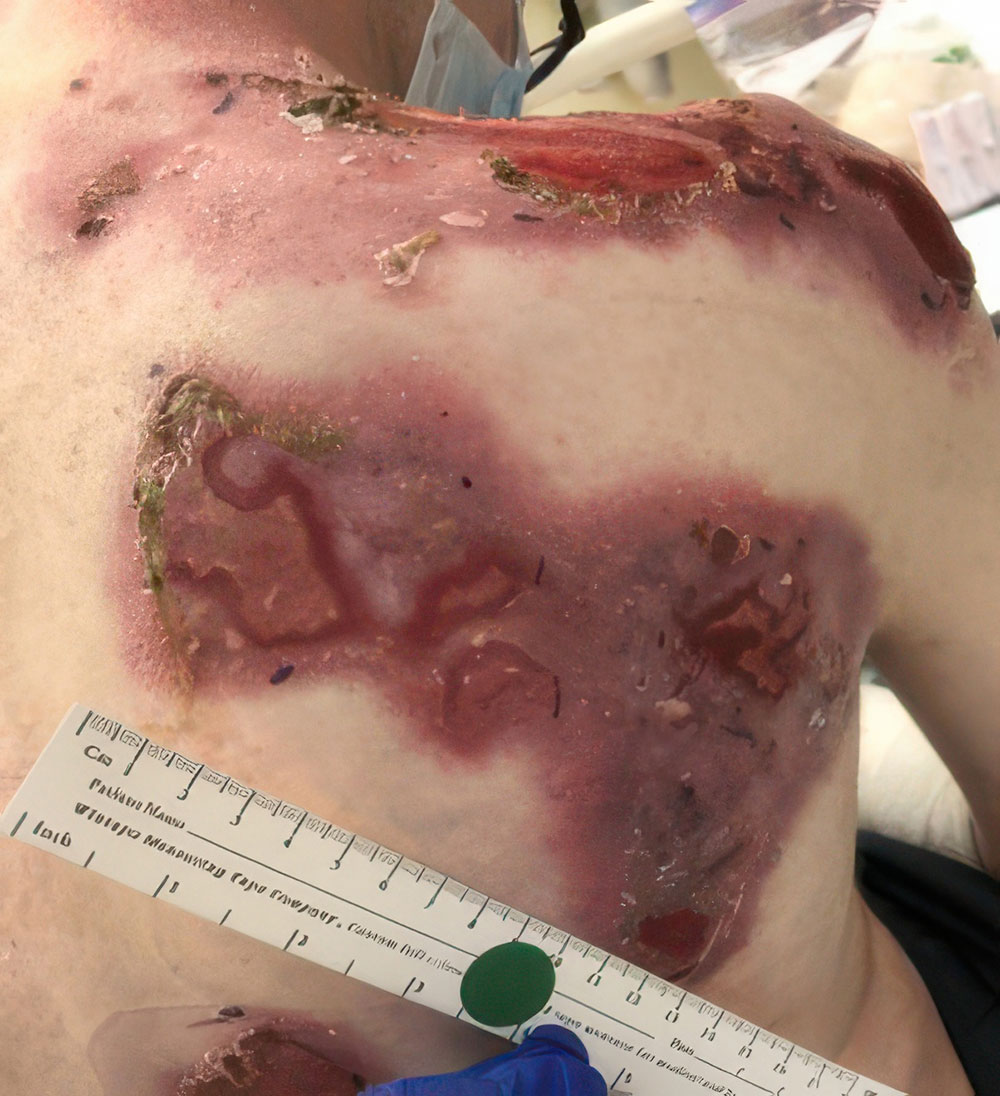
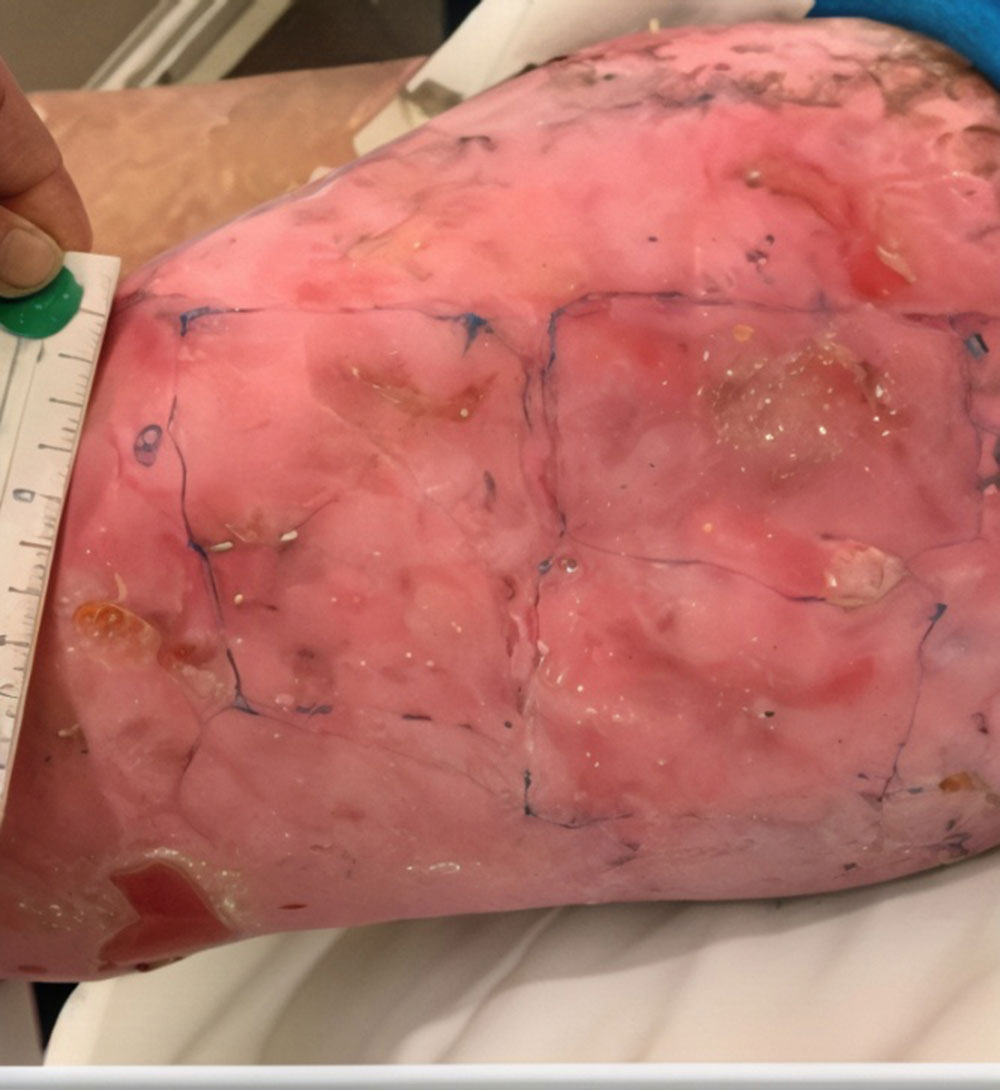
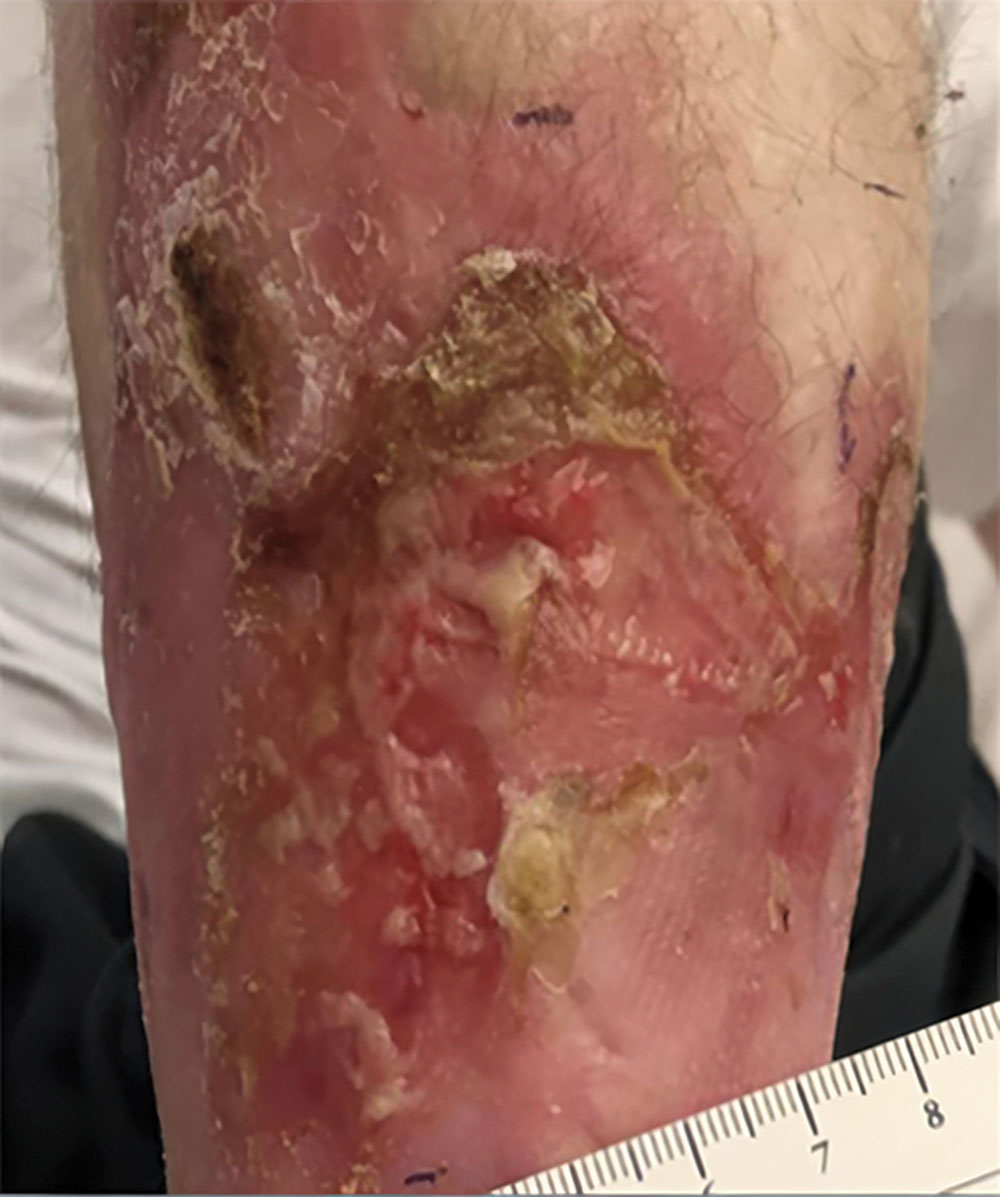
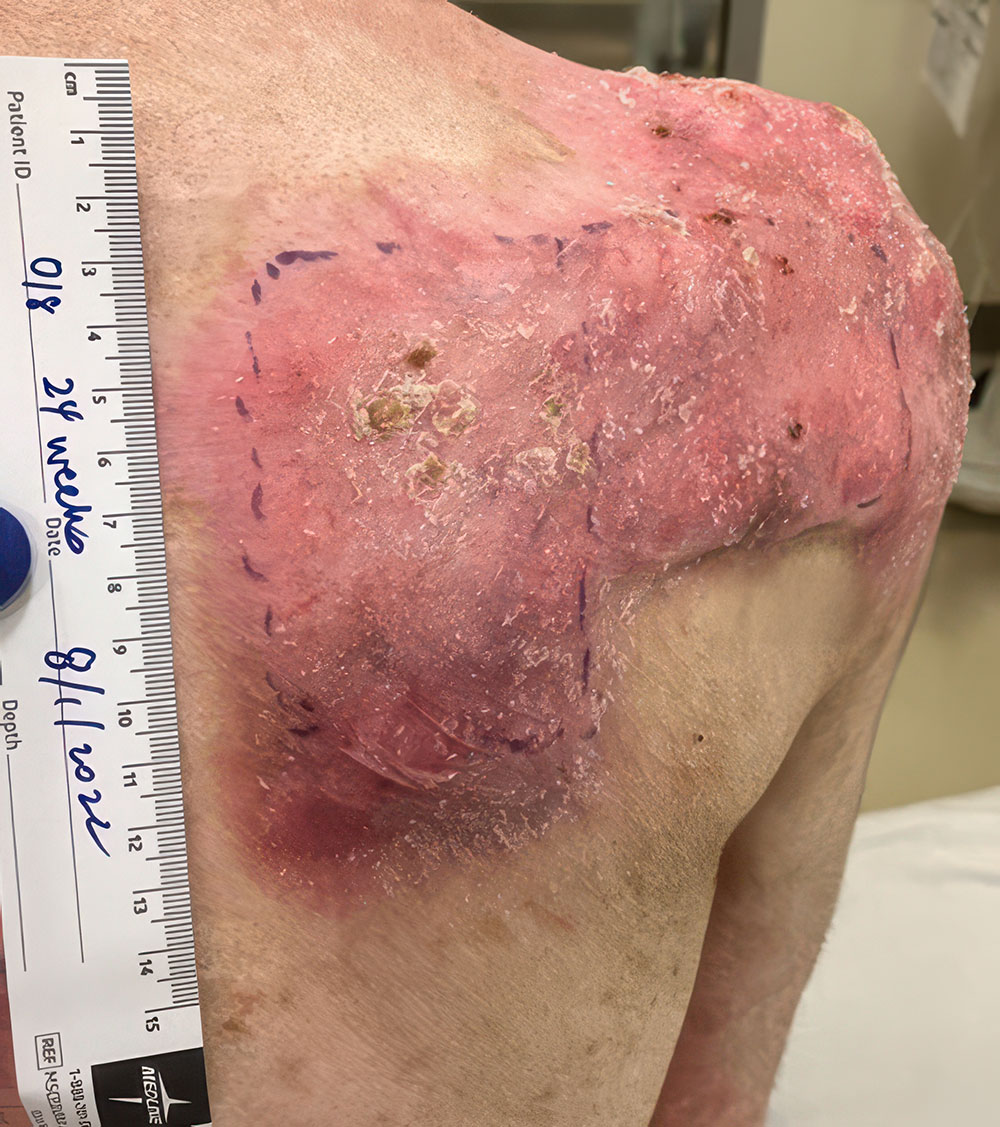
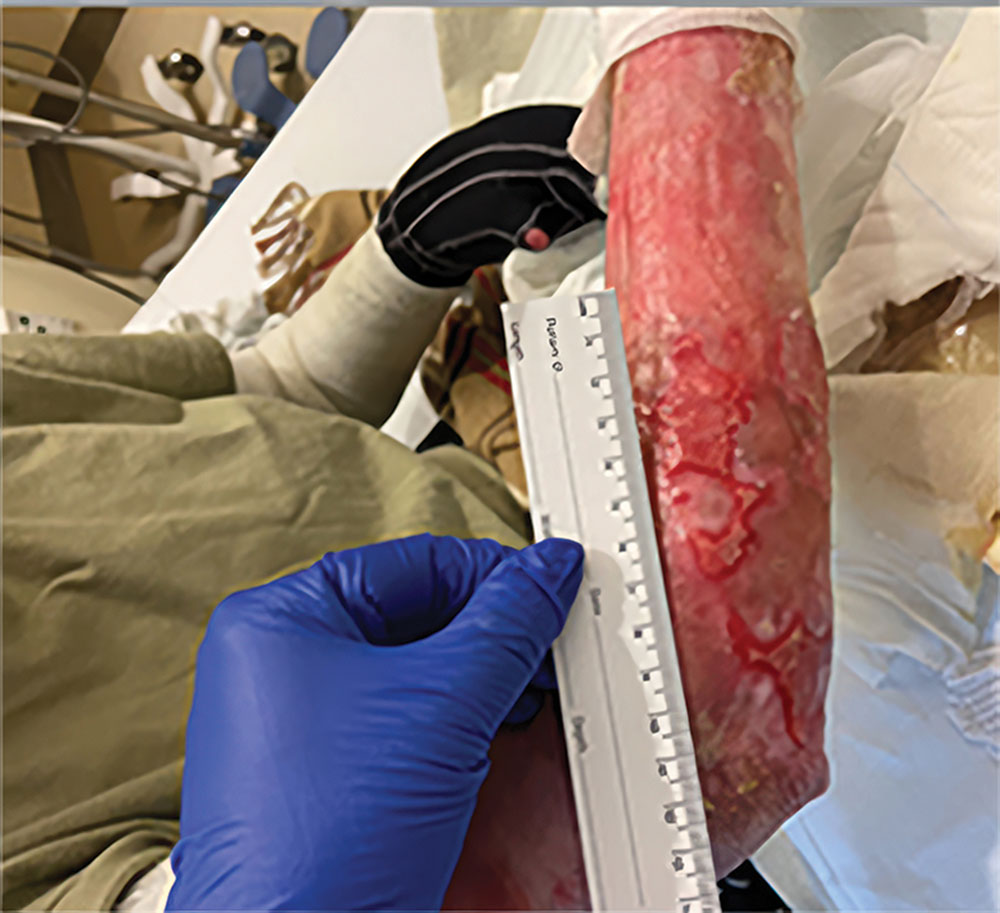
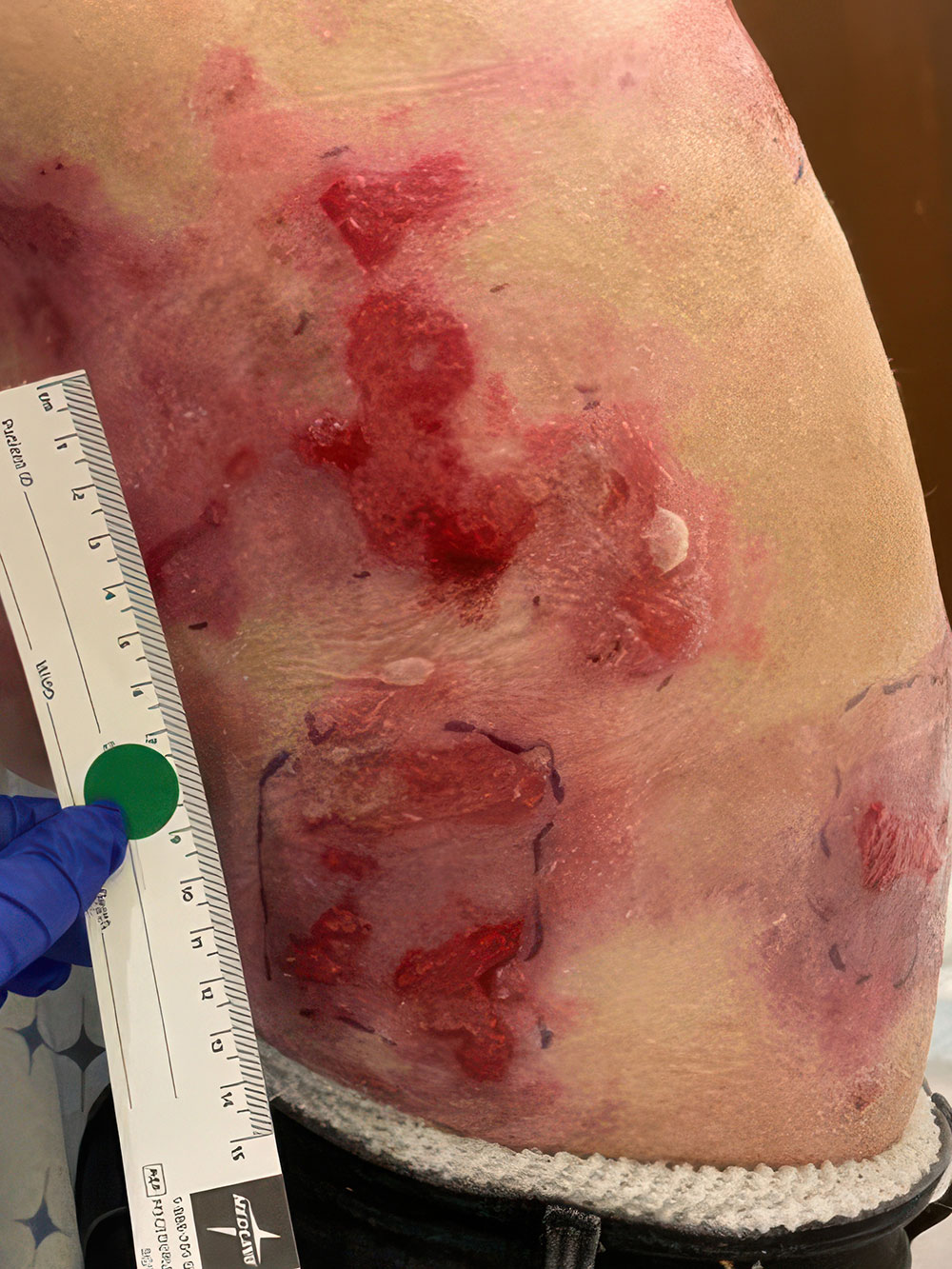
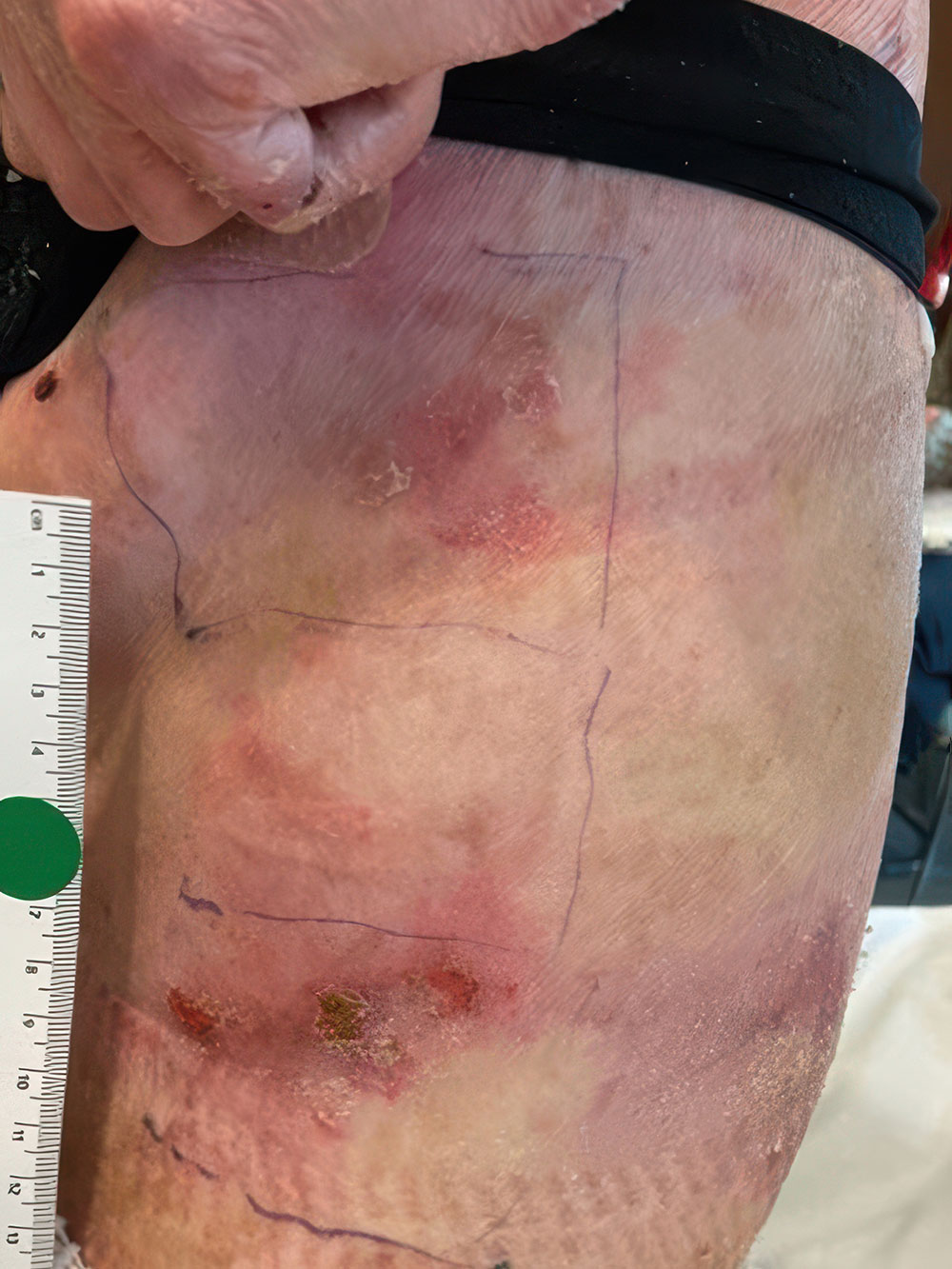
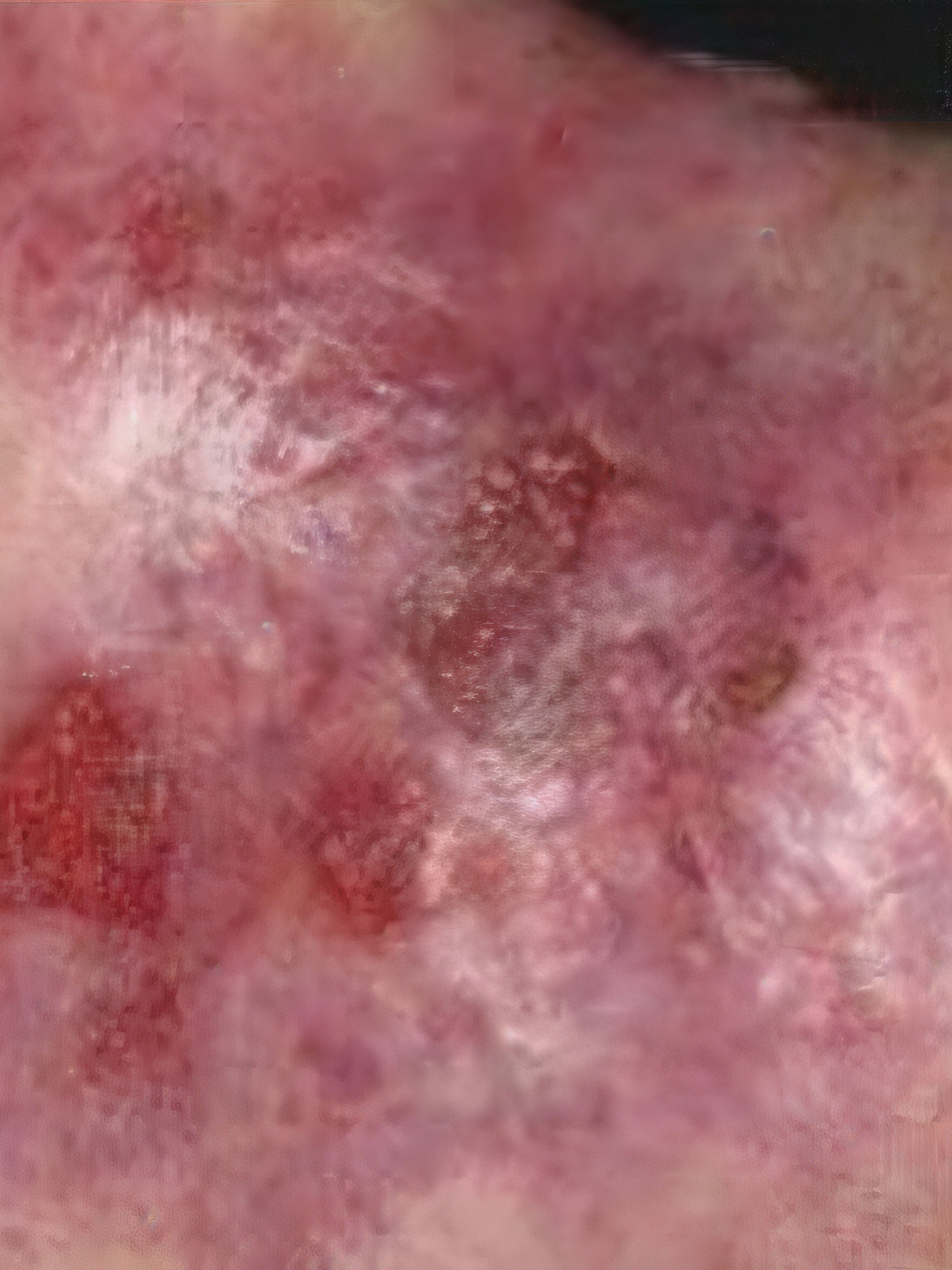
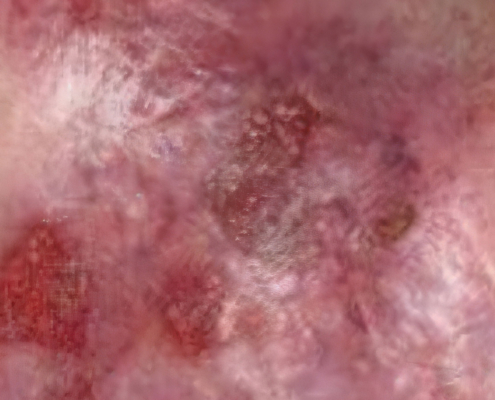
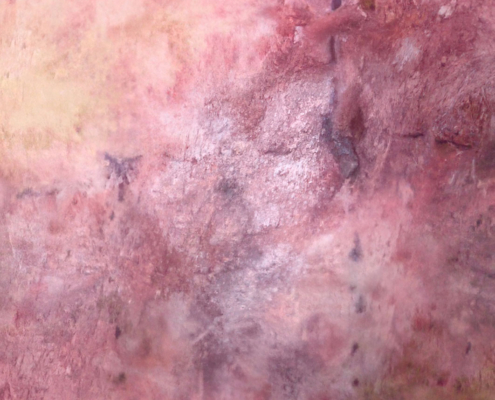
ZEVASKYN can close even tough RDEB wounds
See what healing with ZEVASKYN treatment can look like at 6 months
What are the side effects of ZEVASKYN?
Established safety profile
ZEVASKYN has been evaluated in 43 large, chronic wounds in 11 patients
- The most common adverse reactions occurring in ≥5% of patients were procedural pain (3 of 11 patients; 27%) and itching (1 of 11 patients; 9%)
- No grade 3 side effects were reported#
Serious allergic reactions to ZEVASKYN can occur, including rare cases of anaphylaxis. ZEVASKYN may increase the risk of cancer due to how it works; lifelong monitoring is recommended. It is made from human and animal materials, and although tested, the risk of infection transmission cannot be completely ruled out. Please see additional Important Safety Information below.
- #Grade 3 side effects are considered severe and medically significant, but not immediately life-threatening. They can interfere with daily activities, limit self-care, and may even require or prolong hospitalization.
Additional long-term safety from clinical studies
- In 99 large, chronic ZEVASKYN-treated wounds across 18 patients:
- Median (middle value) follow-up of 3 years and 2 months after treatment (range of 12 months to 11 years)**
- No cases of squamous cell carcinoma (SCC) were reported in ZEVASKYN-treated wounds
- SCC is a type of skin cancer that develops in the epidermis, or outer layer of the skin
- SCC was observed in non-treated sites in 4 patients
- **Median is the middle number in a group of numbers arranged from lowest to highest.

When the nurse and doctor took off the bandage and I saw that new skin, when I saw it I just cried.”
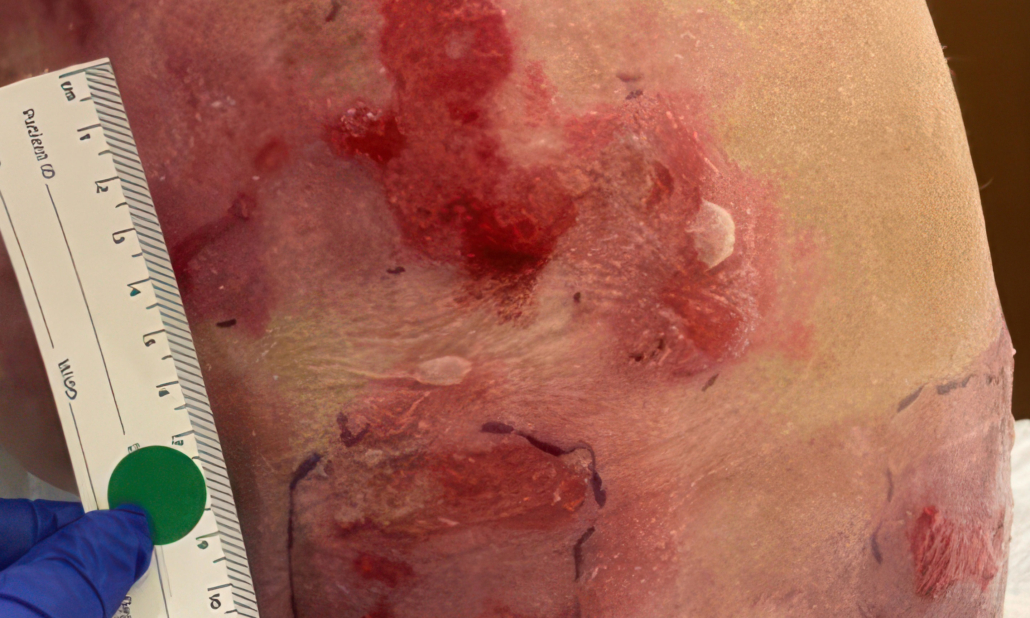
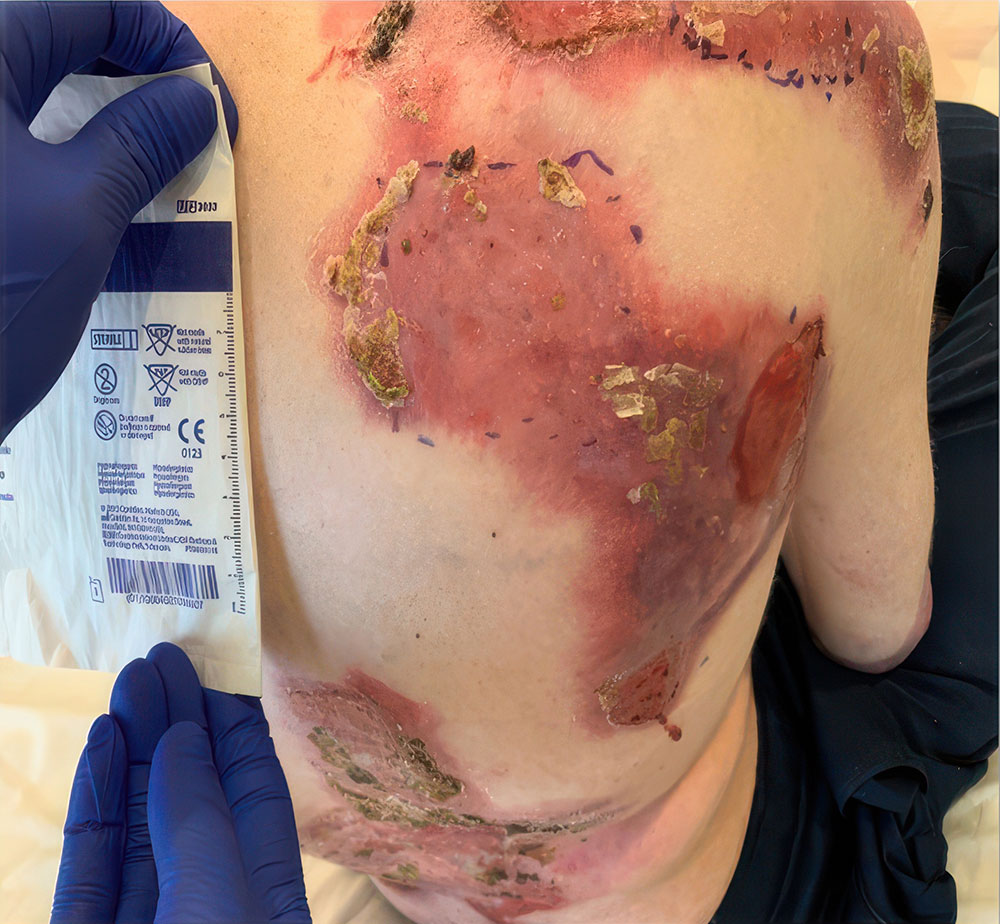
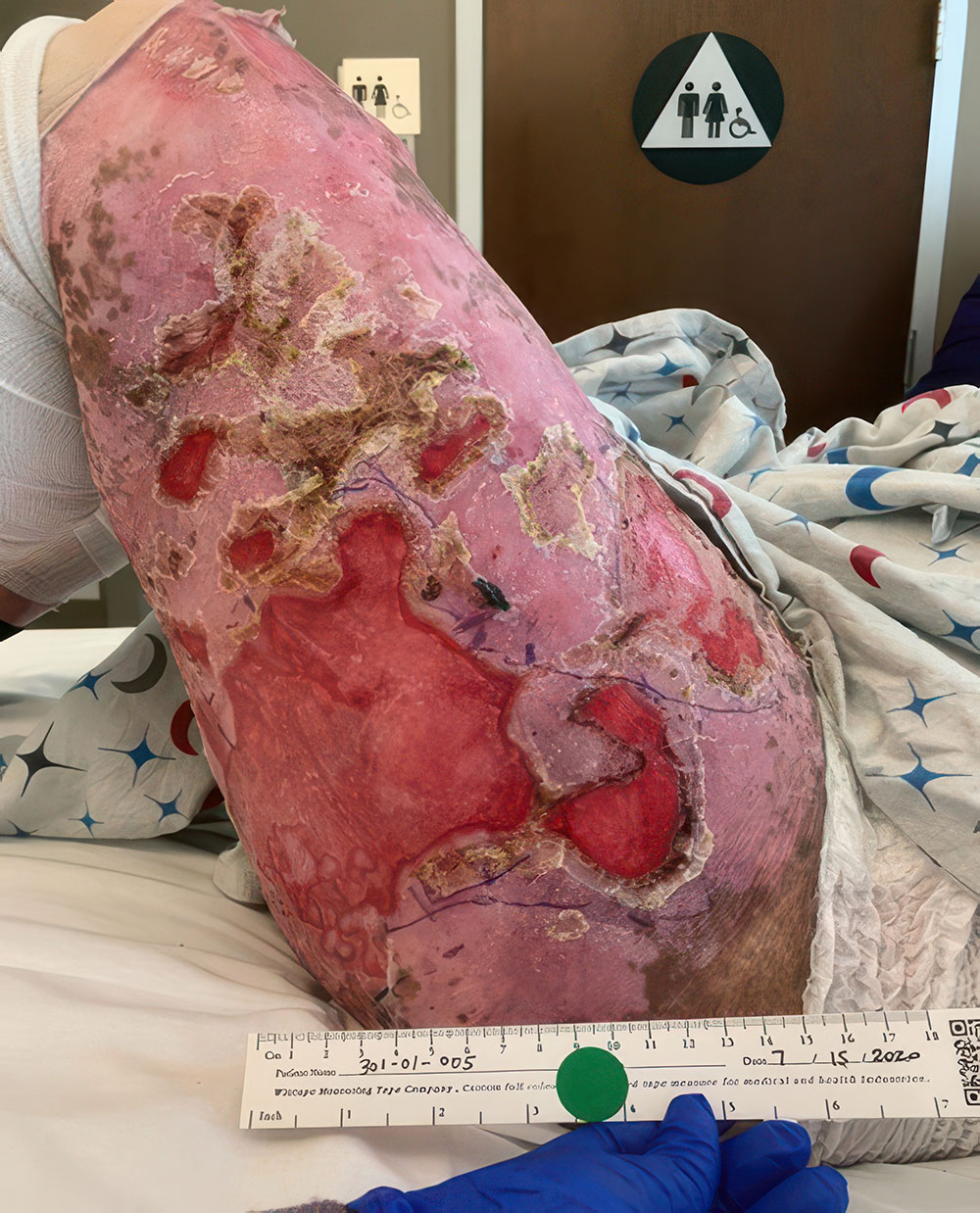
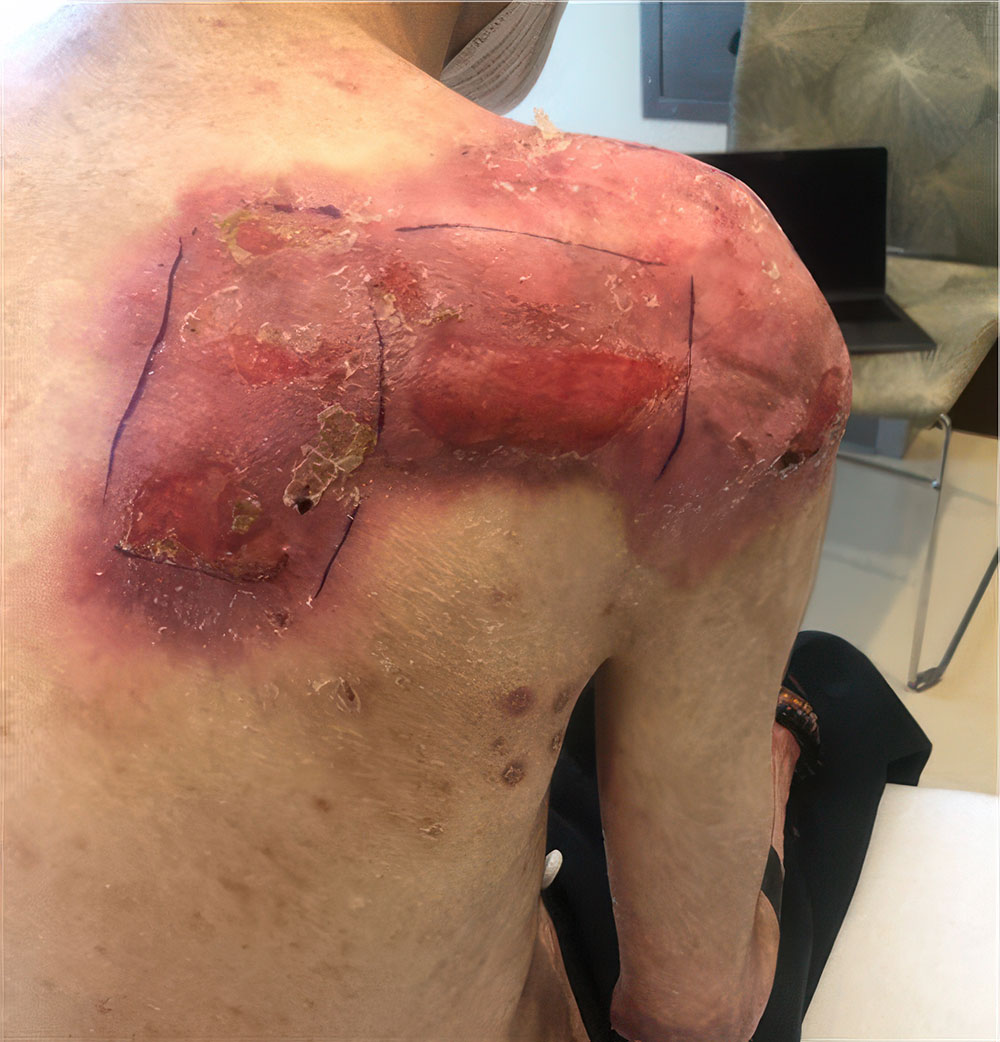
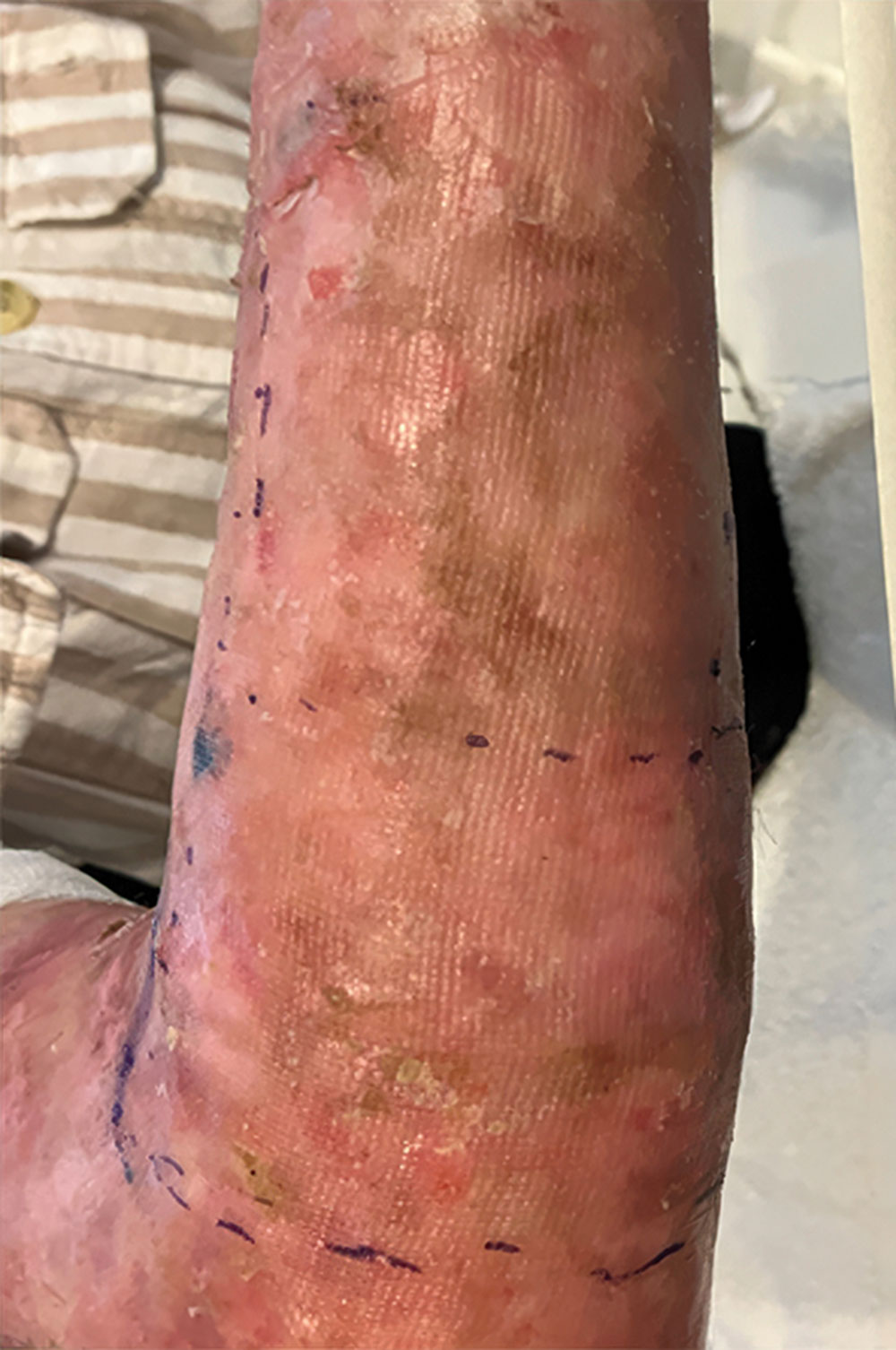
Example of wound healing after long-term follow-up
See what healing with ZEVASKYN can look like long term
In a separate clinical study of 7 patients with RDEB, 38 chronic wounds were assessed long term after treatment with ZEVASKYN. The study did not evaluate ZEVASKYN-treated wounds against control wounds or placebo.
- Patients were followed for a median (middle value) of 6.9 years (range 4-8 years), with a planned follow-up of 15 years††
- Because the study did not include a control group or placebo for comparison, it was not designed to determine whether the observed effects were due to ZEVASKYN.
- ††Median is the middle number in a group of numbers arranged from lowest to highest.